Every day, scientists perform research where glucose measurements are required. These measurements are often done by drawing blood using a handheld glucometer at specified time points. Continuous glucose monitors have become extremely popular for monitoring glucose levels in human patients and are believed to provide a more accurate picture of a person’s glucose profile. Historically, researchers have been unable to obtain continuous data from animal models as no comparable solution was available. However, this is now possible with DSI’s continuous glucose telemetry.
Continuous measurements of glucose are believed to be more translatable to clinical applications as they more closely mirror human experience and offer more data than is possible with intermittent sampling. Telemetry offers numerous benefits to researchers and the animals used in a study. The most important benefits are improved animal welfare, higher quality data, advanced study designs, and, in many cases, cost savings. The following provides an overview of how this solution allows for advanced study designs and includes insight into the benefits gained with continuous glucose telemetry.
1. Animals can be used sequentially as their own control
Doctors Klaas Kramer and Lou Kinter were at the forefront of replacing manual or restrained methods with telemetry in their studies. In a paper published in 2003, they summarized the value of continuous data collected with telemetry:
“The quality of physiological measurements collected from conscious unstressed animals is superior since they are collected under conditions that best represent the normal state of the animal, are least influenced by chemical, stress, and physiological factors and (where appropriate) are most predictive of the results that would be achieved in human beings”.1
Specifically related to glucose research, telemetry often improves studies by requiring less blood to be drawn from animals, less handling, removed need for restraint, and fewer animals to achieve the same statistical power. These improvements may also allow movement from parallel to cross-over study designs (e.g. Latin Square), effectively removing “patient effect”, thereby reducing variability and increasing precision.2
Intermittent blood sampling is the most commonly used method of measuring glucose in animal models. However, there is a limit to how much blood can be drawn from each animal, especially in mice and rats.3,4,5 As a result, many animals are often needed to complete a study. With glucose telemetry, researchers only need to draw 1-3 blood samples per week for calibration purposes while gaining access to continuous data. This often reduces the number of animals needed in a study, allows animals to be used as their own control, reduces the amount of handling required, and allows animals to be used in more than one study.6,7
Animal handling can be a major source of stress for both the animal and laboratory staff.7 Rats and mice experience greater stress from human interaction and it shows in the data. Although implantable telemetry requires a surgical procedure, animals tolerate the implants without issue, and studies show greater than 90% of complete telemetry data sets are free from stress artifact resulting in cleaner, more accurate data.8,9 In addition, some animals, such as non-human primates, can be difficult to work with. Reducing the amount of handling required of laboratory staff greatly decreases their stress as well. As fewer blood draws are needed, there may also be an opportunity for a reduction in laboratory staff, offering a potential source of cost savings.
Ultimately, higher quality data typically result in smaller sample sizes. The opportunity to use animals as their own control and in multiple studies may lead to further reduction. By requiring fewer animals and reducing stress experienced by those used, the use of continuous glucose telemetry honors the reduction and refinement requirements of the 3Rs of animal welfare.10
2. Detect subtle changes which would otherwise be missed
Data are measured every second for a period of at least 4 weeks and frequently 6 to 8 weeks. This allows you to see information you may have missed with intermittent sampling. Intermittent sampling requires you to make an educated guess on timing of samples, and it is unknown what may have occurred between each time point.
In addition to blood glucose, body temperature and activity are collected continuously, allowing correlation among glucose levels, temperature and activity at all times. This correlation provides a better understanding of the animal’s whole physiologic profile.
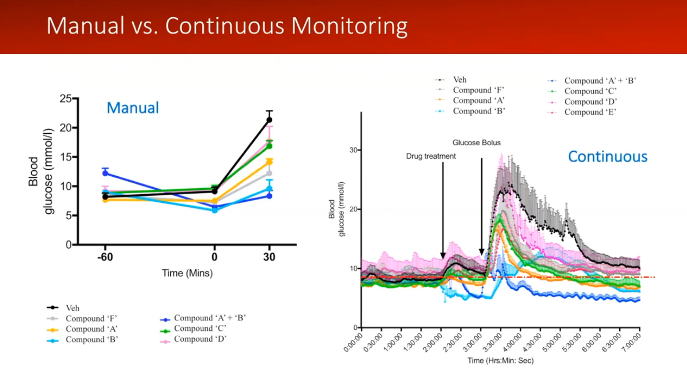
Dr. Stephanie Simonds from Monash University showed the difference continuous data made in her research in a recent webinar with Inside Scientific.11 Dr. Simonds discussed the difference in conducting a glucose tolerance test (GTT) with intermittent sampling and continuous glucose telemetry. When you perform a manual GTT, you can see the changes in glucose levels. However, you are not able to see the exact time points of these changes or what may occur between samples. As shown in Dr. Simonds’ slide below, there is a drastic difference in the amount of data provided. With continuous data, you have a complete picture of what the animal experiences throughout the study. Dr. Simonds said:
“I don’t think you get that information when you do a manual analysis. So you don’t really understand what your drug is doing until you have this kind of information… and put it together with the rest of the physiology that’s going on.”
3. Opportunity for quantification of new and improved biomarkers
New biomarkers can be quantified with continuous blood glucose data which are not possible with intermittent sampling. For example, when intermittent samples are spaced far apart (e.g. weekly or once daily) the researcher has no visibility of what happens between samples, and glucose homeostasis must be inferred. Continuous glucose telemetry allows quantification of glucose homeostasis including assessments of averages, standard deviations, glycemic variability and more. With continuous data, you know what happens every second, minute, or hour of every day. Essentially, you won’t miss a thing and will have the ability to analyze anything of interest.
Existing biomarker calculations can also be more robust by using continuous data. Examples include using data prior to human presence and animal handling for calculation of fasting plasma glucose and using data throughout a GTT to enhance calculation and accuracy of area under the curve (AUC). Further, if the fasting plasma glucose level is more accurately assessed, then whenever it is used as the baseline for the AUC calculation, that biomarker will also be improved. Baseline assessments and area over the curve calculations would be similarly improved in insulin tolerance tests.
Watch the video below to go through a case study showing how continuous glucose data can be used and the benefits you could gain by integrating it into your research.

This video is part of an upcoming video series on DSI’s continuous glucose solutions. Keep an eye out for additional videos coming soon to our website!
If you would like to learn more about continuous glucose or receive a free benefits evaluation to get a personalized look at how it could impact your research, complete our request form.
References
1Kramer K, Kinter LB. Evaluation and applications of radiotelemetry in small laboratory animals. Physiological Genomics 2003;13(3):197–205. doi: 10.1152/physiolgenomics.00164.2002
2Sarazan, RD, Telemetry in Nonclinical Drug Safety Assessment Studies. In Gad SC(Ed), Animal Models in Toxicology, Third Edition. CRC Press 2016 pp. 877-894.
3NIH Office of Animal Care and Use. Guidelines for Survival Bleeding of Mice and Rats. August 12, 2015. Retrieved from https://oacu.oir.nih.gov/sites/default/files/uploads/arac-guidelines/rodent_bleeding.pdf
4McGuill MW, Rowan AN. Biological Effects of Blood Loss: Implications for Sampling Volumes and Techniques. ILAR News 1989;31(4):5-18. doi:10.1093/ilar.31.4.5
5BVA/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Removal of blood from laboratory mammals and birds. First report. Lab Anim 1993;27:1-22.
6Ping X, Yao Z, Nawrocki A, Doerning B, Johnson C, Shen X. Telemetry for Continuous Glucose Monitoring in Rats. Poster P296, AALAS 67th National Meeting, Charlotte, NC, October 30-November 3, 2015
7Handling and Restraint, https://www.nc3rs.org.uk/handling-and-restraint. Accessed 13 Feb 2017
8Moran, M. M., R. R. Roy, C. E. Wade, et al. 1998. Size constraints of telemeters in rats. J. Appl. Physiol. 85(4):1564–1571.
9Baumans, V., J. A. Bouwknecht, H. Boere, et al. Intraperitoneal transmitter implantation in mice: Effects on behavioural parameters and bodyweight. Medicinal chemistry. 1999 Vol. 56, p.23-24
10Morton DB, Hawkins P, Bevan R, Heath K, Kirkwood J, Pearce P, Scott L, Whelan G, Webb A, Refinements in telemetry procedures: Seventh report of BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement, Part A, Laboratory Animals, 2003;37(4):261-299. doi: 10.1258/002367703322389861
11From Mouse to Monkey: Revolutionizing Research via Preclinical Continuous Glucose Telemetry. Webinar July 2018. https://insidescientific.com/webinar/mouse-to-monkey-preclinical-continuous-glucose-telemetry-DSI
Papers of interest related to telemetry:
- El Amrani A, El Amrani-Callens F, Loriot S, Singh P, Forster R. QT interval correction for drug-induced changes in body temperature during integrated cardiovascular safety assessment in regulatory toxicology studies in dogs: A case study, Journal of Pharmacological and Toxicological Methods 2016;81:136–143. doi: 10.1016/j.vascn.2016.04.008
- FDA. ICH Harmonized Tripartite Guideline, S7A Safety Pharmacology Studies for Human Pharmaceuticals. 2001. Available at: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM074959.pdf
- Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. AHA Scientific Statement: Recommendations for Blood Pressure Measurement in Humans and Experimental Animals. Part 2: Blood Pressure Measurement in Experimental Animals. Hypertension 2005;45:299-310. doi: 10.1161/01.HYP.0000150857.39919.cb
Papers of interest related to glucose:
- Hirsch IB, Brownlee M. Should minimal blood glucose variability become the gold standard of glycemic control? Journal of Diabetes and its Complications 2005; 19(3):178–181. doi: 10.1016/j.jdiacomp.2004.10.001
- Service FJ. Glucose Variability. Diabetes May 2013;62:1398-1404. doi:10.2337/db12-1396
- Siegelaar SE, Holleman F, Hoekstra J, DeVries JH, Glucose Variability; Does It Matter? Endocr Rev 2010;31(2):171-182. doi:10.1210/er.2009-0021
- Bergenstal RM, et al. Recommendations for standardizing glucose reporting and analysis to optimize clinical decision making in diabetes: the Ambulatory Glucose Profile (AGP). Diabetes Technol Ther March 2013;15(3):198-211. doi: 10.1089/dia.2013.0051. Epub 2013 Feb 28.
- Mazze RS, et al. Characterizing Glucose Exposure for Individuals with Normal Glucose Tolerance Using Continuous Glucose Monitoring and Ambulatory Glucose Profile Analysis. Diabetes Technol Ther May 2008;10(3):149-159. doi:10.1089/dia.2007.0293
- Marling CR, Shubrook JH, Vernier SJ, Wiley MT, Schwartz FL. Characterizing Blood Glucose Variability Using New Metrics with Continuous Glucose Monitoring Data. Journal of Diabetes Science and Technology 2011;5(4):871-878. doi:10.1177/193229681100500408
- 21Nathan DM, et al. Translating the A1C Assay Into Estimated Average Glucose Values. Diabetes Care Aug 2008;31(8):1473-1478. doi:10.2337/dc08-0545